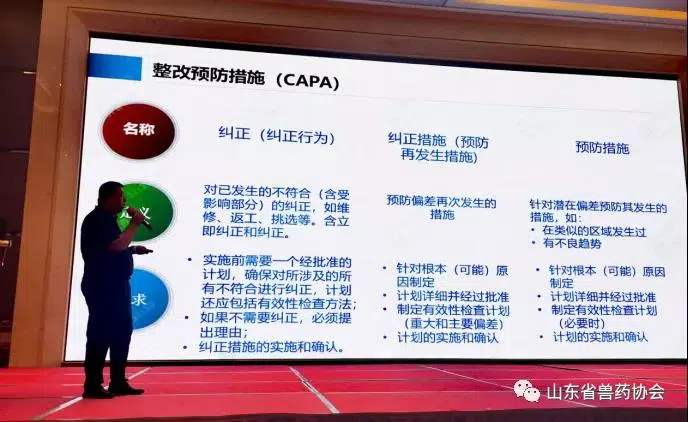
Shandong Veterinary Drug Association Held A New Version Of Veterinary Drug GMP Training Meeting
On September 13, 2021, Shandong Veterinary Drug Association held a new version of veterinary drug GMP training meeting in Jinan. Zhang Feng, the CEO of Zhengzhou Vtops Machinery Co Ltd, was invited to participate in this meeting.

We learned from organizer, more than 540 technicians and quality managers attended the training meeting.Including veterinary drug manufacturers managers, production managers, quality managers and other related personnel from Shandong province.
Some of veterinary drug related agencies also participated in this training meeting are following:
- Shandong Provincial Animal Product Quality Inspection Center.
- Shandong Provincial Feed veterinary Drug Quality Inspection Center.
- Jinan Agricultural Product Quality Inspection Center.
Some of scholars and researchers of veterinary drug related participated in this training meeting are following:
- Duan Wenlong, Researcher and Chief Reviewer of China Institute of Veterinary Drug Supervision.
- Liu Guohua, Director of Policy and Regulations Department of Shandong Animal Husbandry and Veterinary Bureau.
- Fang Wei, Researcher of Feed and Veterinary Drug Department.
- Shao Bing, Director of Shandong Animal Product Quality Inspection Center.
- Yang Zhikun, Deputy Director of Shandong Feed and Veterinary Drug Quality Inspection Center.
The training aims to promote the smooth implementation of the new veterinary drug GMP in Shandong provice. Accelerate the transformation and upgrading of enterprises. Enhance the competitiveness of veterinary drug products. Promote the healthy and high-quality development of animal husbandry. Ensure food safety and public health safety.
What is the GMP?
GMP is a mandatory standards for the pharmaceutical manufacturers, food industries and other industries. It requiring companies following relevant regulations of the state to meet the health quality requirements. Especially on raw materials, personnel, facilities, equipment, production process, packaging, transportation, quality control, etc. It formed a set of operational norms to help enterprises improve the enterprise sanitation environment. And discover the problems existing in the production process and solve timely.
In brief, GMP requires pharmaceutical and food manufacturers should have the system to ensure the final product quality. Including food safety and health to meets regulatory requirements. The system including the good production equipments, reasonable production process, perfect quality management, strict testing system and so on.
The purpose of GMP are the following:
1, Cross Pollution: avoid the pollution and cross pollution in the process of drug production to the maximum extent.
2, Improve Quality: reduce the occurrence of various errors, is an important measure to improve the quality of drugs.
Why Should Build a New Version of GMP?
The establishment of GMP in standards with international standards. It helps to promote the mutual recognition of GMP inspection results between countries. In other words, only entering the orbit of international standard, it has infinite market space of extension. For this purpose, the new version of GMP takes examples by the international advanced standards and drug regulatory experience. It pays more attentions to science and focuses on refining the software requirements. But also it puts forward higher technical requirements in the hardware, especially the sterile drugs part. The standard has a high starting point and better guarantee for drug production control and production quality.
The new veterinary drug GMP puts forward higher request to standardize the production behavior of veterinary drug manufacturers. Especially on the veterinary biological products and sterile veterinary drug production standard. The request such as, the enterprise’s factory building, facilities, equipment, etc. All veterinary drug manufacturers need to conduct technical analysis and research on existing and upcoming pharmaceutical equipment and packaging equipment. And conduct transformation and upgrading according to their own economic capacity and product process requirements. To ensure that the enterprise hardware meets the requirements of the new GMP specification.
The new GMP implementation ensure the more safety of millions of people’s drug use legally. It is a challenge for the majority of Chinese drug manufacturers, but also a rare opportunity. It is the starting point for enterprises to eliminate backwardness, realizing upgrading and flying on a higher platform.
Promote of MARA
The MARA (Ministry of Agriculture and Rural Affairs of China) moreover issued a series of supporting documents to promote the implementation of the new veterinary GMP after it issuance. Starting from June 1, 2020, new veterinary drug manufacturing enterprises, as well as veterinary drug manufacturing enterprises’ renovation, expansion, or relocation and reconstruction of production workshops, shall meet the requirements of the new version of veterinary drug GMP [1]. However, all of veterinary drug manufacturers have two-year buffer period, requiring comply with the new veterinary drug GMP requirements by May 31, 2022. The transformation not only a heavy task but also the time pressing. Therefore, the veterinary drug manufacturers should make an active plan. Formulate implementation plans and organize implementation according to their own realities.
For this purpose, all of pharmaceutical-related companies should be fully preparing. Due to this, Zhengzhou Vtops Machinery Co Ltd has released many improvements and applied many patents on our packaging machines. These include: how to improve accuracy, how to clean quickly, how to prevent cross-contamination, and so on.
Vtops Report on The New GMP Training Meeting
Li Mingchong made a speech at the new Version of GMP Training Meeting of Shandong Province.
Mr. Li Mingchong, the co-founder of Zhengzhou Vtops Machiney Co Ltd. He made an speech providing intelligent production and packaging solutions in line with GMP for veterinary drug manufacturers. And he shared with everyone the report “How to realize high-efficiency dust-free packaging of the Veterinary medicine powder, premix, multi-variety powder, multi-bag type, multi-specification “. Mr. Li Mingchong’s speech was highly recognized and praised by the organizers, and also pointed out the direction and provided effective solutions for the GMP process of participating enterprises.
Vtops have professional level in the field of packaging automation has been continuously improved. Vtops have successfully developed a variety of material batching packaging machine. Such as, auger filling machine, VFFS packaging machine, PFS packaging machine and other packaging machinery equipments. They have been widely used in food, medicine, chemical, agriculture and many other fields.
In addition, VTOPS has obtained more than many technology patents and invention patents. But also we have a self building 40,000 square meters of modern industrial plants.
References:
[1] No. 293 announcement of Ministry of Agriculture and Rural Affairs of China: http://www.moa.gov.cn/gk/tzgg_1/gg/202005/t20200506_6342920.htm
[2] 3D rendering of VTOPS factory, please click: https://www.vtops.com/3d/.